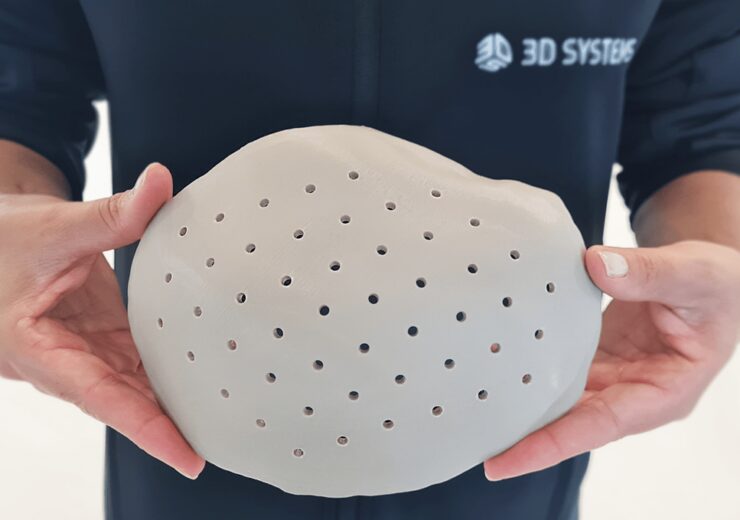
Nota3D, a leading provider and reseller of 3D printing technologies, is thrilled to announce 3D Systems has officially gotten FDA Approval for the PEEK Cranial Implant. This groundbreaking medical device, produced using the EXT 220 MED 3D printer, represents a significant advancement in patient-specific cranial reconstruction.
The FDA-approved PEEK Cranial Implant enables the creation of tailor-made solutions for skull defects, offering superior biocompatibility, resistance to bodily fluids, and excellent performance characteristics closely mimicking human bone. This approval allows Nota3D to deliver cutting-edge, efficient, and safe medical devices directly to U.S. healthcare providers.
“We are excited to offer this state-of-the-art solution, which significantly enhances the surgical outcomes for patients requiring cranial reconstruction,” said Jason Jasosch, CEO of Nota3D. “This approval is not just a milestone for 3D Systems and Nota3D but for the entire medical community, advancing how we approach personalized medicine.”
For further information on how to access the PEEK Cranial Implant, please visit www.nota3d.com.
About Nota3D
Nota3D specializes in the distribution and support of leading 3D printing technologies aimed at transforming medical, industrial, and commercial applications. By partnering with pioneering manufacturers like 3D Systems, Nota3D ensures the delivery of top-tier additive manufacturing solutions globally.